Overview
Brain dysfunction has a detrimental effect on our daily lives. At the same time, we still know surprisingly little about the underlying causes of these neuropathological disorders. We are interested to discover how our brain maintains its remarkable function of learning and memorizing with the constant exposure to environmental cues that might imbalance the neuronal network and lead to a disease phenotype.
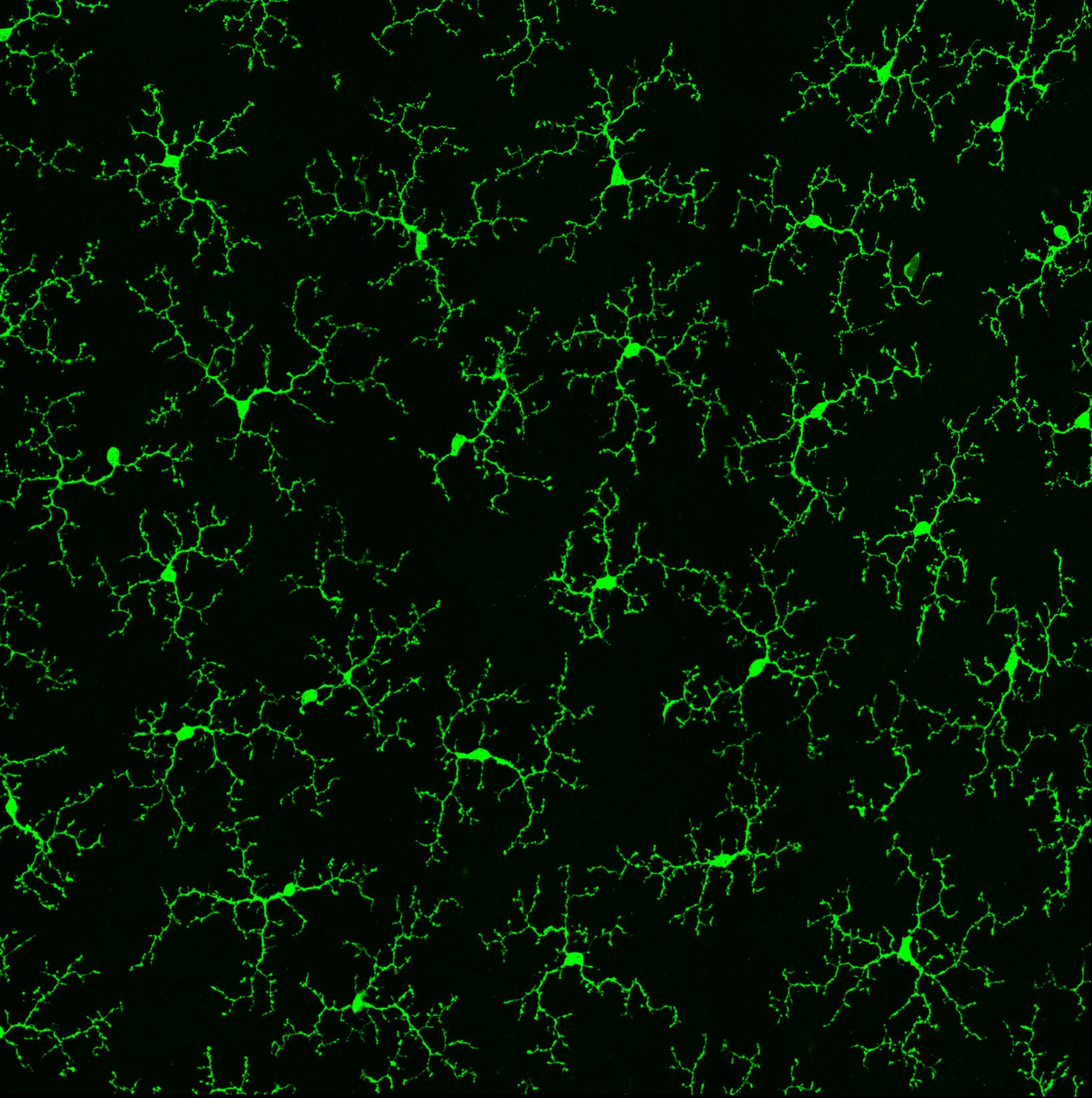
However, neurons are surrounded by neuroglia, a term that incorporates all non-neuronal cells in the brain. These cells are commonly seen as only supporting neuronal tissue function but they also express several disease-associated genes and therefore entered the spotlight as a potential source of disease with microglia at the forefront. Microglia have been shown to respond to environmental cues and at the same time are actively involved in disease onset and progression, in which they remove synapses and extracellular matrix. This raises the question, if microglia cannot respond properly or are distracted in their communication to other cell types in response to an environmental stimulus, how will this impact the functionality of the neuronal network?
Poor communication causes tension between different parties increasing the number of conflicts and ruining relationships, if not resolved in time. We anticipate that microglia are a key element for triggering neuronal circuit maladaptation leading to psychiatric and neurodegenerative disease phenotypes.
Connecting our knowledge from virology, immunology, molecular, cellular, behavior neuroscience, and epigenetics together with concepts of applied mathematics and machine learning puts us in a unique position to address the microglia role in the nervous system.
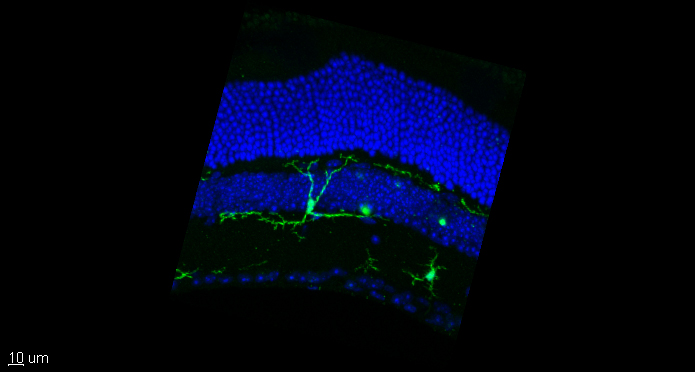
As our model system, we are taking advantage of defined brain regions amongst other the retina, and human induced pluripotent stem cells (hiPSCs) to model human-related aspects.
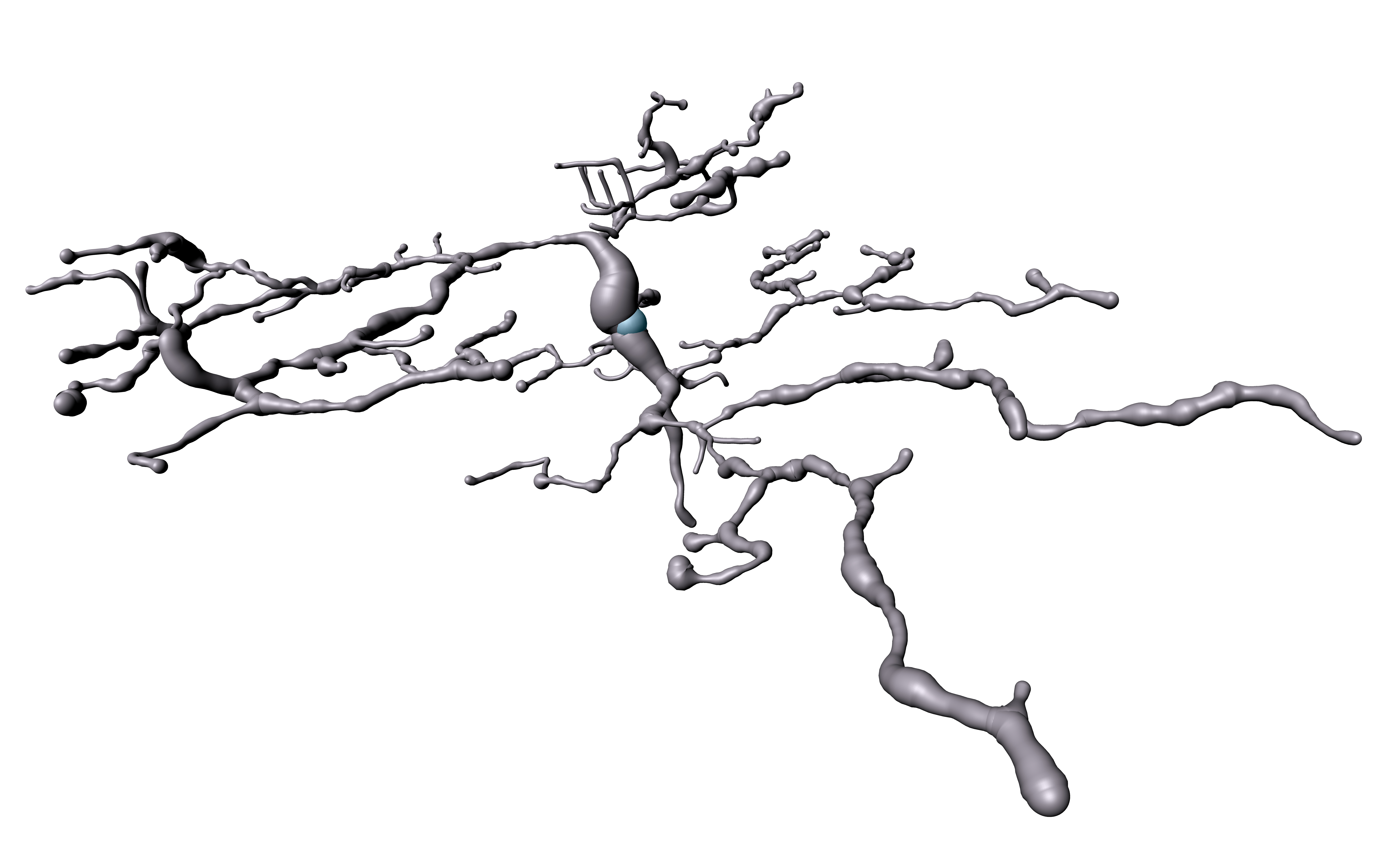
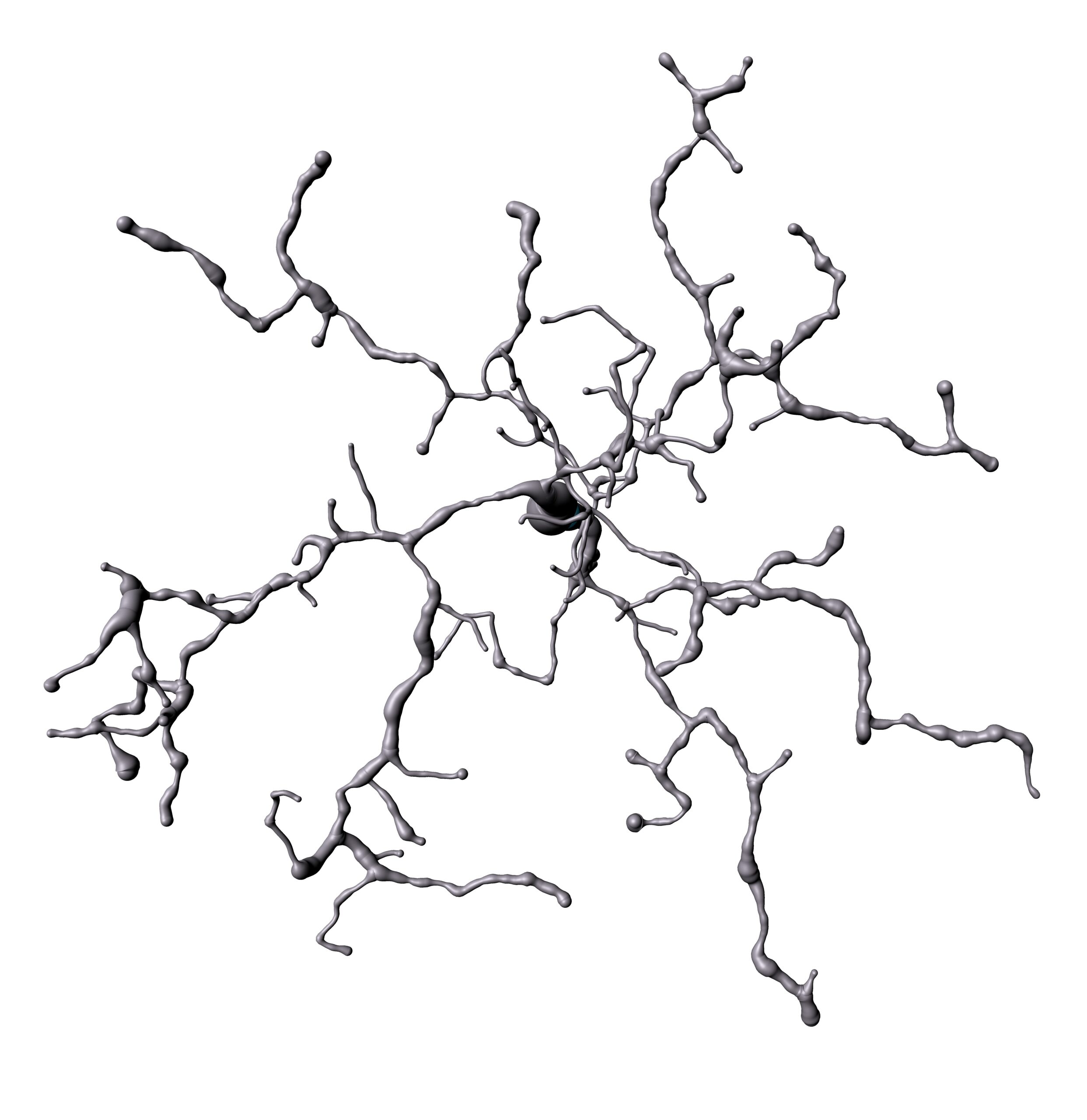
So far, we have shown that upon repeated exposure to anesthetic ketamine microglia are enabled in a widespread cortical perineuronal net loss and reinstatement of juvenile-like plasticity in adult mice (Venturino et al. 2021). This surprising effect should be reflected in obvious morphological adaptations however, morphological strategies are less well suited to do so. Thus, we have developed a multifaceted, interdisciplinary strategy named MorphOMICs, which resolves differences in microglia morphology based on brain region, sex, developmental stage and disease progression (Colombo et al. 2022). We generated an extensive morphological atlas, which can be unlimitedly extended with other microglial cue-dependent phenotypes such under dietary changes, stress, sleep and pharmacological interventions, which will guide future hypothesis-driven approach.
In parallel, we have established strategies to manipulate microglia function e.g., using viral approaches in the retina (Maes et al. 2021), or manipulating G protein-coupled receptors, a class of receptors extensively expressed in microglia (Schulz et al. 2022). Our chimeric DREADD provides a new advancement to selectively drive canonical and non-canonical pathways to dissect the endogenous function of the diverse GPCRs-types in microglia.
Finally, we generated human induced pluripotent stem cells (hiPSCs)-derived microglia and retinal organoids and identified microglia-like cells in the mesenchymal niches (Bartalska et al. 2022).
In our on-going research, we are focusing on the following research topics:
- To disentangle the relationship between microglia structure and function.
- To identify how environmental cues drive microglia to modulate circuit formation and function. Specifically, we are focusing here on:
- How microglia manipulation affects individual circuit components?
- How microglia and the neuronal network adapt to repeated, minimal-invasive insults?
How microglia inflammatory responses affect human neuronal circuit formation?